A few months before the first COVID-19 vaccines received Emergency Use Authorization EUA in late 2020 a global vaccine safety expert cautioned the rushed circumstances made it essential to get safety monitoring right. Latest data on the safety and effectiveness of COVID-19 vaccines in children Childhood vaccinations provide immunity to children against serious infectious diseases before they are exposed to them to prevent illness.
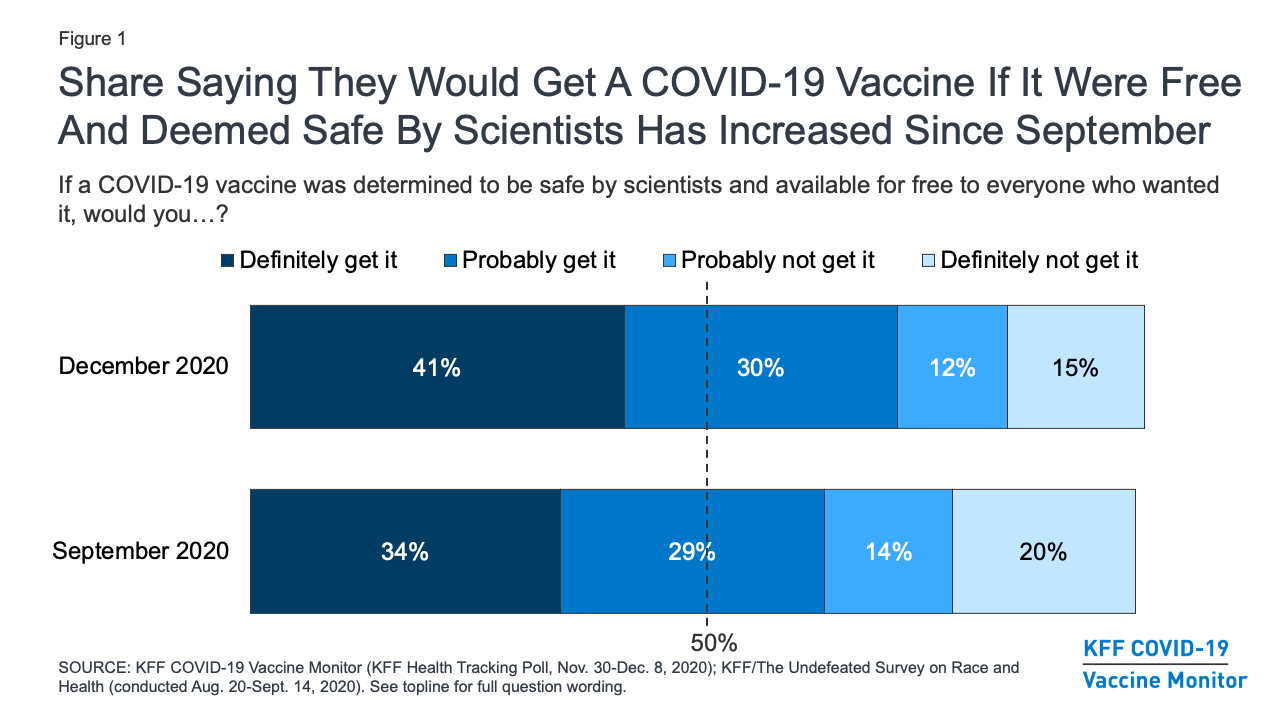
Kff Covid 19 Vaccine Monitor December 2020 Kff
Safety data for shots rolling out in other parts of the world such as the COVID-19 vaccines in China are harder to come by.
Covid vaccine safety data. A few months before the first COVID-19 vaccines received Emergency Use Authorization EUA in late 2020 a global vaccine safety expert cautioned the rushed circumstances made it essential to get safety monitoring right by intensively and robustly scrutinizing adverse events following the experimental rollout. April 2 2021 Over 334 million doses of COVID-19 vaccine have been given in the United States from December 14 2020 through July 12 2021. As this expert stated Deploying any new vaccine based on.
COVID Data Tracker as of Feb 16 2021 107571 doses with manufacturer not identifiedSelf-reported during a v-safehealth check-in 6 Gee J Marquez P Su J et al. The most common reactions reported for the AstraZeneca COVID-19 vaccine in the week of 19-25 July 2021 were headache fever muscle pain fatigue and nausea. All three types of vaccines are safe and effective in preventing serious cases of COVID-19.
The most common reactions reported for the Pfizer Comirnaty COVID-19 vaccine in the week of 12-18 July 2021 were headache muscle pain fatigue dizziness and nausea. First Month of COVID-19 Vaccine Safety Monitoring United States December 14 2020January. On April 23 CDC advisers recommended that use of the JJ vaccine restart with a label stating the risk of the condition.
How Do We Know They Are Safe. The most common reactions reported for the Comirnaty Pfizer COVID-19 vaccine in the week of 19-25 July 2021 were headache muscle pain fatigue dizziness and nausea. Ninety percent of adults in America will be eligible for a COVID-19 vaccine by April 19 according to the White House.
54 cases received COVISHIELDAstraZeneca COVID-19 vaccines three received a Pfizer vaccine and one received a Moderna vaccine. COVID-19 Vaccine Safety and Efficacy Data Overview. EUAs were given for mRNA vaccines.
After vaccines are authorized and in use by the public public health officials continue monitoring the data as an additional safety measure. MRNA Covid-19 Vaccine Safety in Pregnant Persons Preliminary data from the CDC v-safe after vaccination health checker surveillance system the v-safe pregnancy registry and the Vaccine. The FDA issued emergency use authorizations EAU for three vaccines.
Symptoms started between 1 and 34 days after vaccination. The FDA granted emergency use authorization EUA because research data from large clinical trials has shown them to be safe and effective. Pfizer Moderna COVID vaccines face new safety probe in Europe over possible link to skin condition 2 kidney disorders by Kevin Dunleavy Aug 11 2021 233pm.
3rd COVID vaccine dose safe data shows With over 100 people now seriously ill with the coronavirus Health Ministry calls number of people hospitalized with virus worrying If officials find vaccine efficacy declines over time third dose may be discussed ministry says. Data shows far fewer hospitalized from vaccine than COVID-19 Berenson asserts the number of youths hospitalized from COVID-19 vaccinations is four times higher than those from COVID-19. COVID-19 vaccines are safe and effective.
Safety data indicate the investigational vaccine was generally well-tolerated. COVID-19 vaccines were evaluated in tens of thousands of participants in clinical trials. Of the 54 TTS cases following a COVISHIELDAstraZeneca vaccine.
Preliminary data from clinical trials of the adenovirus-based Sputnik V. In people at high risk of developing complications from COVID-19 people 65 years or older and people under age 65 with certain comorbidities or with likely regular exposure to COVID-19 the vaccine showed 910 efficacy in preventing symptomatic COVID-19 disease. The Pfizer and Moderna vaccines authorized by the FDA have very good safety records.
Manufacturers must have a plan to report follow-up data including any events such as hospitalizations and deaths and they must continue research to generate more data on safety and efficacy. Be sure to stay in touch with the news that matters by subscribing to our top news of the day. SAFETY DATA SHEET _____ Product Name Pfizer-BioNTech COVID-19 Vaccine Page 2 12 Revision date 19-Mar-2021 Version 2 of the potential risk.
Your needs may vary depending upon the. The Defender is experiencing censorship on many social channels. The most common reactions reported for the AstraZeneca COVID-19 vaccine in the week of 12-18 July 2021 were headache fever muscle pain fatigue and chills.
This means that millions of people will have the chance to line up for their shot in less than three weeks. The precautionary statements and warnings included may not apply in all cases.

Covid 19 Vaccine Side Effects Aefis And Safety

Safety And Efficacy Of Single Dose Ad26 Cov2 S Vaccine Against Covid 19 Nejm
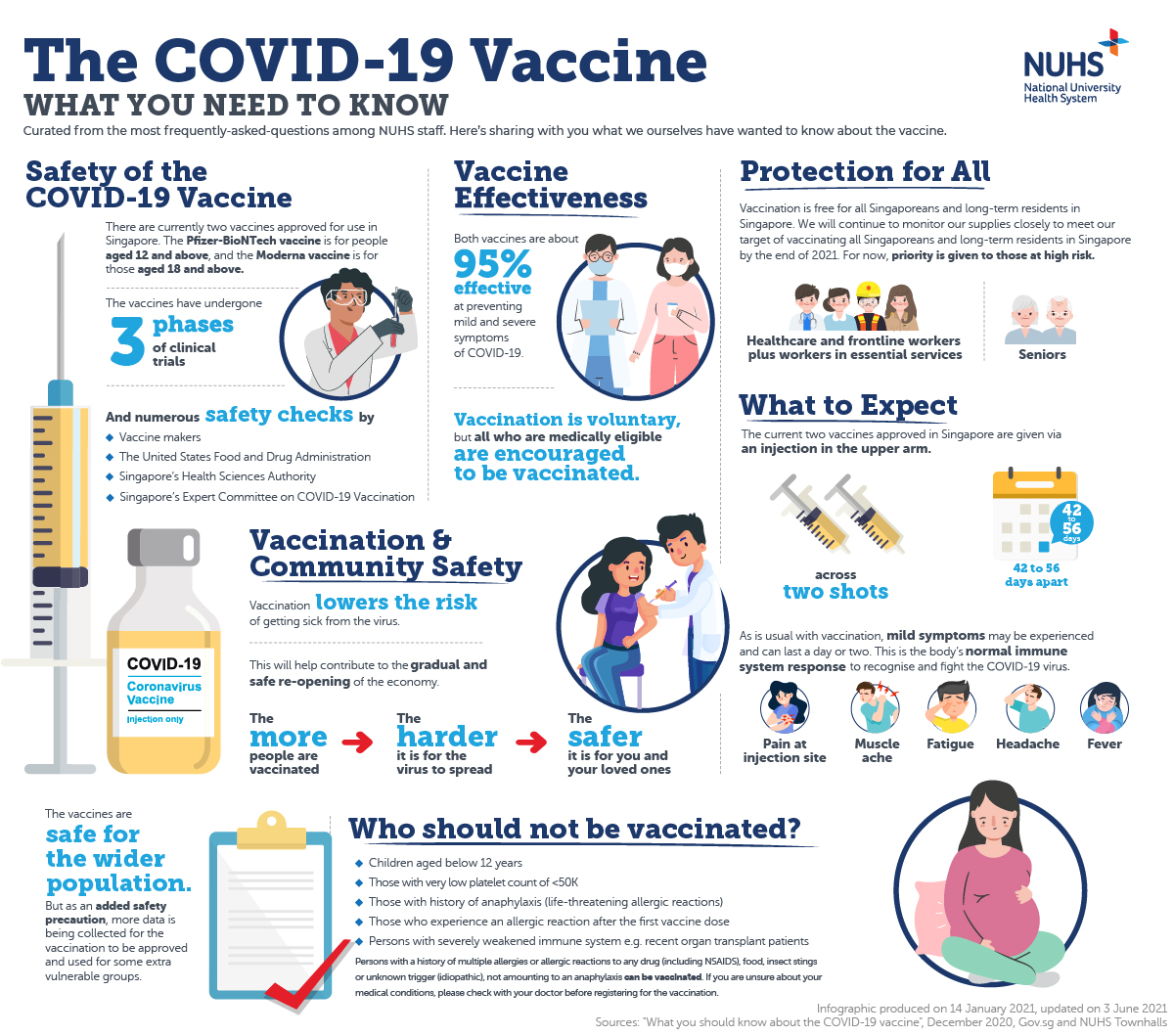
Covid 19 Vaccination Updates Nuhs National University Health System
Covid 19 Vaccines Safety Surveillance Manual

Latest U S Authorizes Booster Shot For People With Compromised Immune Systems

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 14 08 2021

Covid 19 Vaccines Verifying Safety And Identifying Misinformation Covid 19 Johns Hopkins Bloomberg School Of Public Health
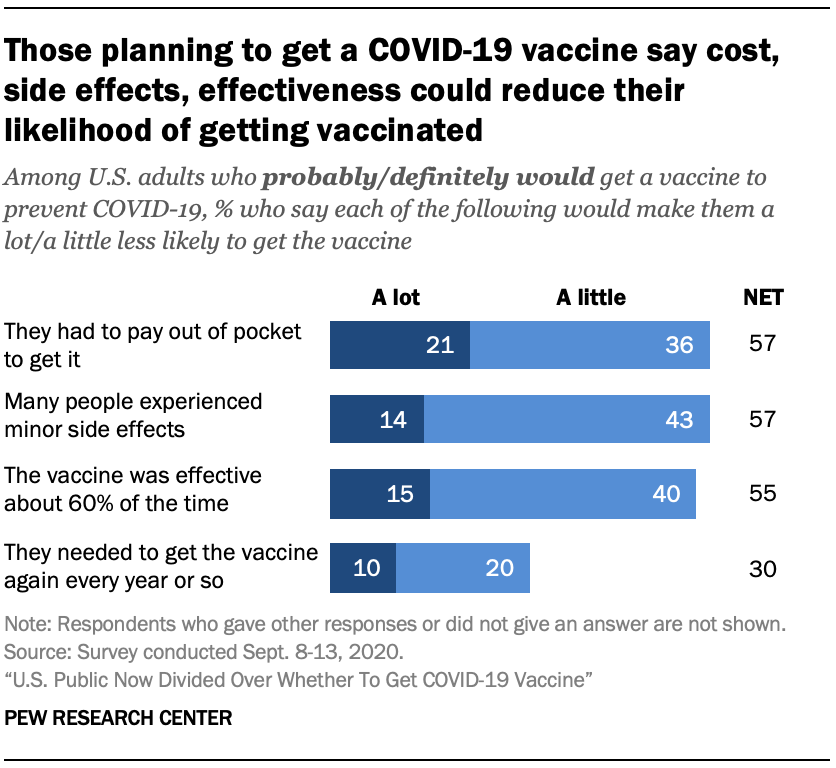
U S Public Now Divided Over Whether To Get Covid 19 Vaccine Pew Research Center

Covid 19 Vaccine Side Effects Aefis And Safety

Covid 19 Vaccines Vaccine Knowledge
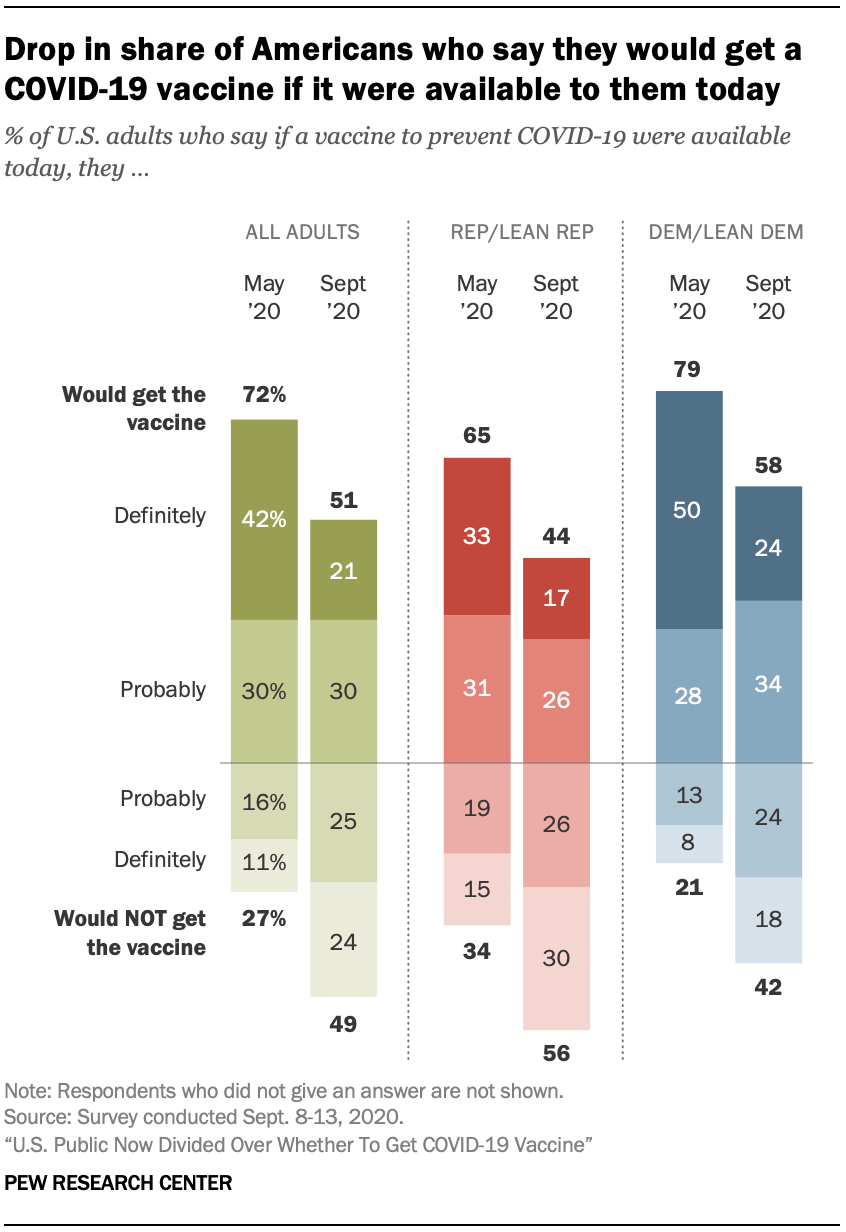
U S Public Now Divided Over Whether To Get Covid 19 Vaccine Pew Research Center
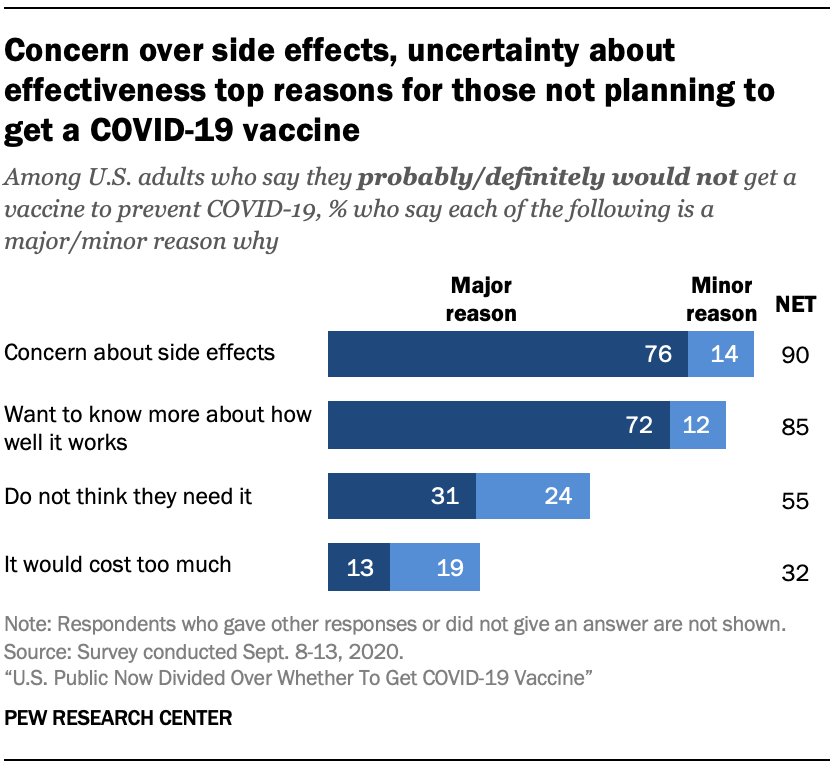
U S Public Now Divided Over Whether To Get Covid 19 Vaccine Pew Research Center

Covid 19 Vaccination Updates Nuhs National University Health System
Https Www Ema Europa Eu Documents Covid 19 Vaccine Safety Update Covid 19 Vaccine Safety Update Comirnaty 11 May 2021 En Pdf

Germany Restricts Use Of Astrazeneca Vaccine To Over 60s In Most Cases News Dw 30 03 2021

The Long View On Covid 19 Vaccine Safety And Efficacy Penn Today
Https Www Fda Gov Media 144327 Download

Covid 19 Vaccination Updates Nuhs National University Health System
Https Www Ema Europa Eu Documents Covid 19 Vaccine Safety Update Covid 19 Vaccine Safety Update Covid 19 Vaccine Janssen 11 May 2021 En Pdf

Post a Comment
Post a Comment